- Article
- Source: Campus Sanofi
- 23 Oct 2023
Head to Head Studies
.jpg)

This is intended for HCPs practising in Great Britain (England, Scotland, Wales) only.
Head-to-head studies Admelog® vs Humalog®
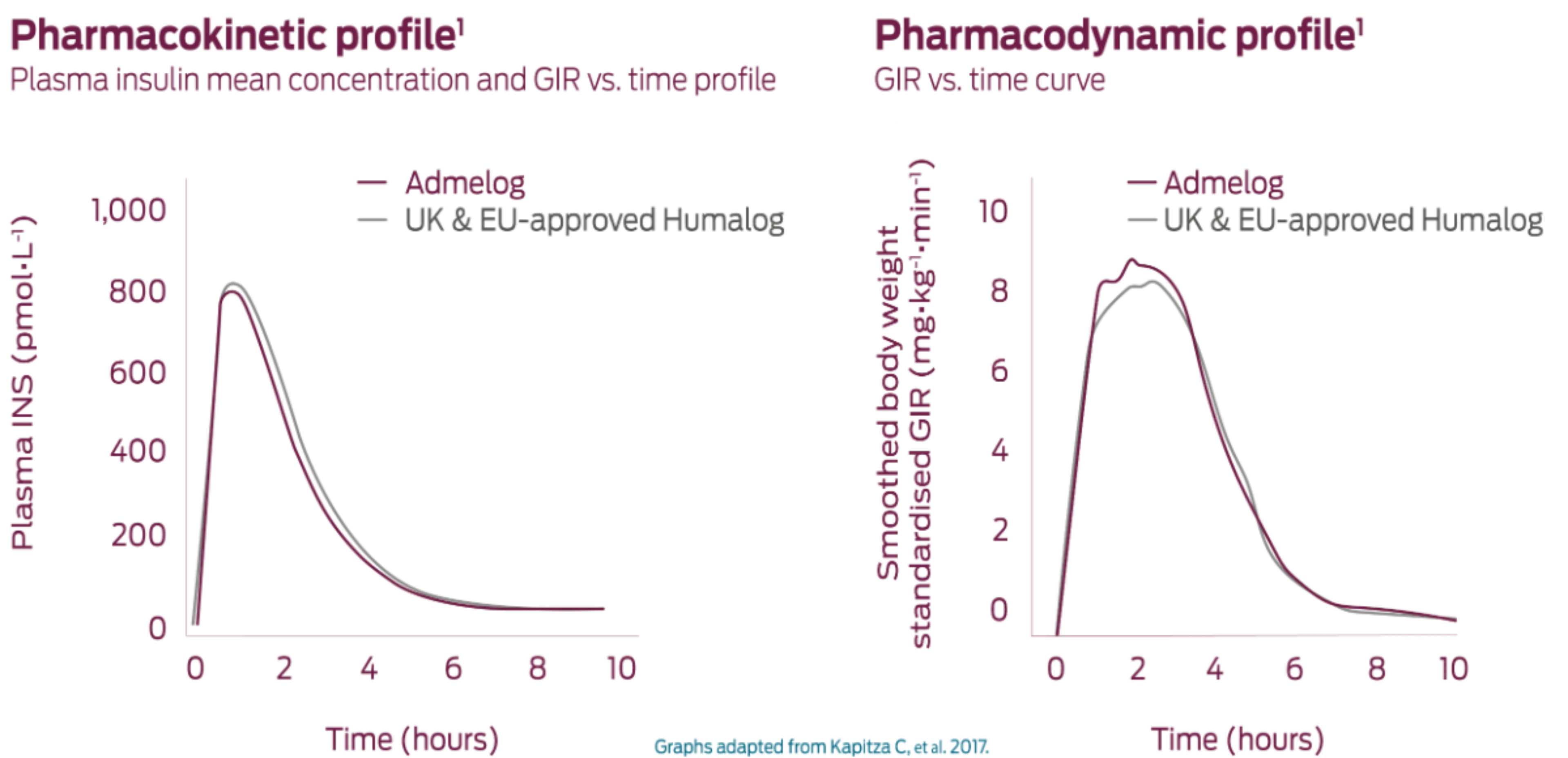
Similar PK/PD profile1
PK/PD profiles in the biosimilar, Admelog, were similar to the EU-approved Humalog in adult males with Type 1 diabetes1
.jpg)
Similar mean HbA1C reduction2,3
Similar mean HbA1C reduction from baseline to Week 26 in Admelog and Humalog groups, in both Type 1 and Type 2 diabetes2,3
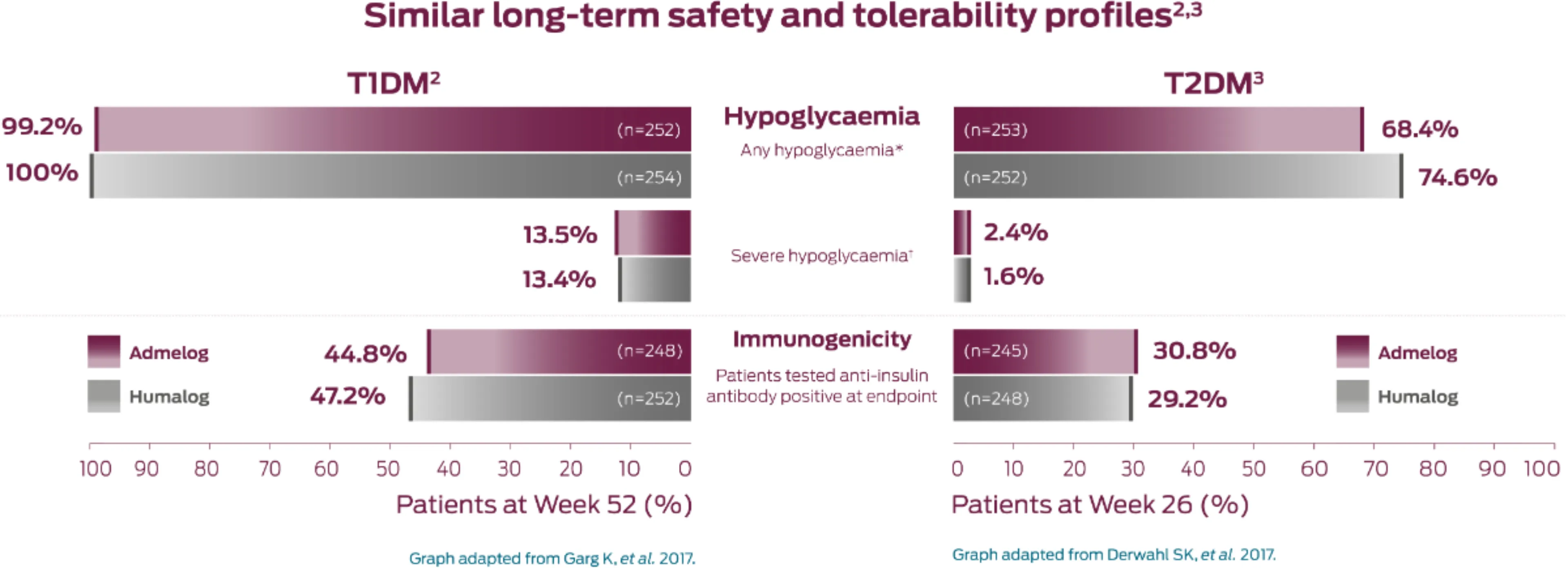
Similar long-term safety and tolerability profiles2,3
Similar rates of patients with at least one hypoglycaemic event, regardless of the category, was observed in both Admelog and Humalog groups in Type 1 and Type 2 diabetes2,3
Abbreviations:
CI, confidence interval; EU, European Union; GIR, glucose infusion rate; HbA1c, glycated haemoglobin; INS, plasma insulin lispro concentration; PD, pharmacodynamic; PK, pharmacokinetic; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; UK, United Kingdom.
Admelog
Admelog is indicated for the treatment of adults and children with diabetes mellitus who require insulin for the maintenance of normal glucose homeostasis and for the initial stabilisation of diabetes mellitus.
This is intended for HCPs practising in Great Britain (England, Scotland, Wales) only.

.jpg)
References
- Kapitza C, et al. Diabetes Obes Metab. 2017;19(5):622–7
- Garg SK, et al. Diabetes Technol Ther. 2017;19(9):516–26
- Derwahl K, et al. Diabetes Technol Ther. 2018;20(1):1–10
MAT-XU-2300438 (v5.0) Date of Preparation: October 2023